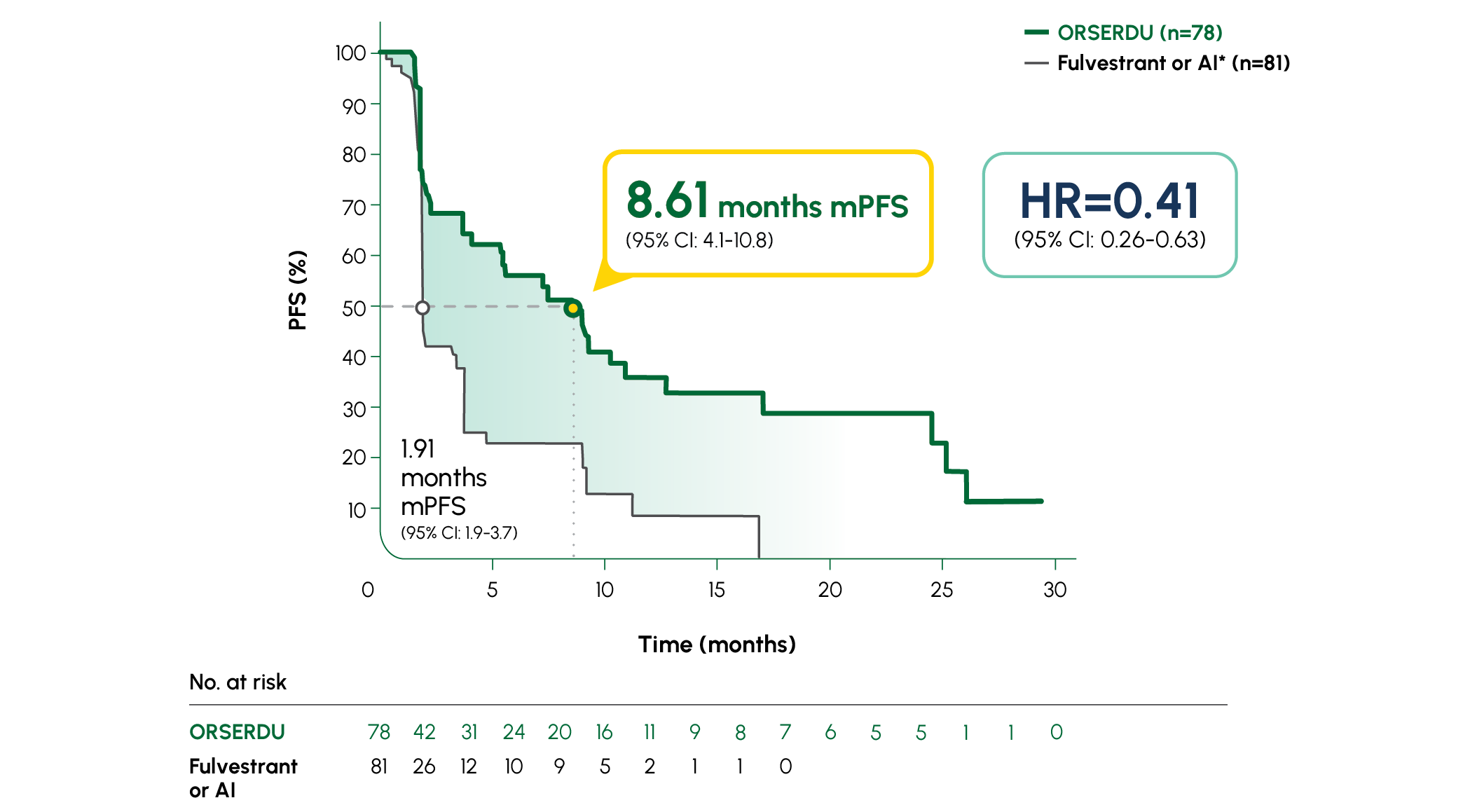
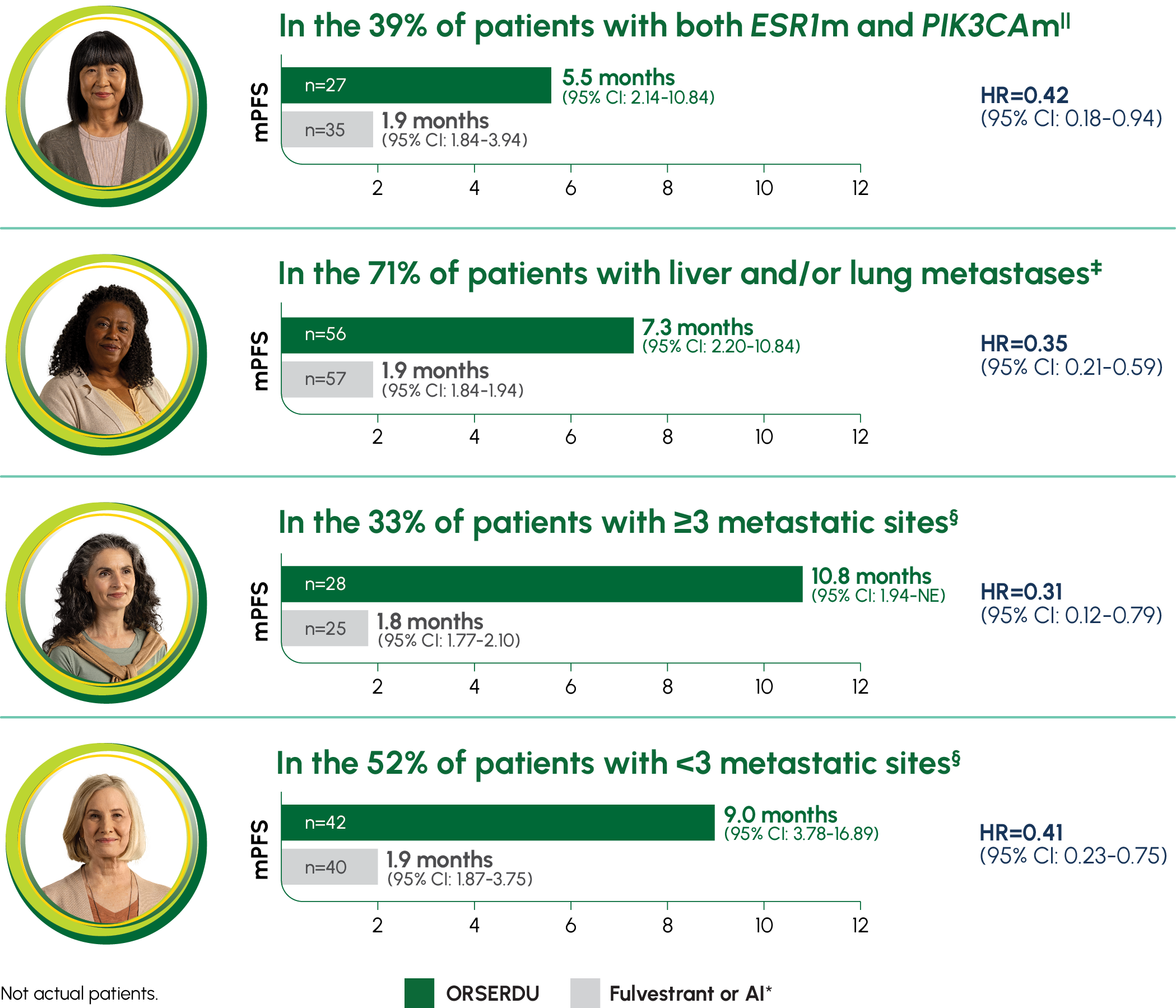
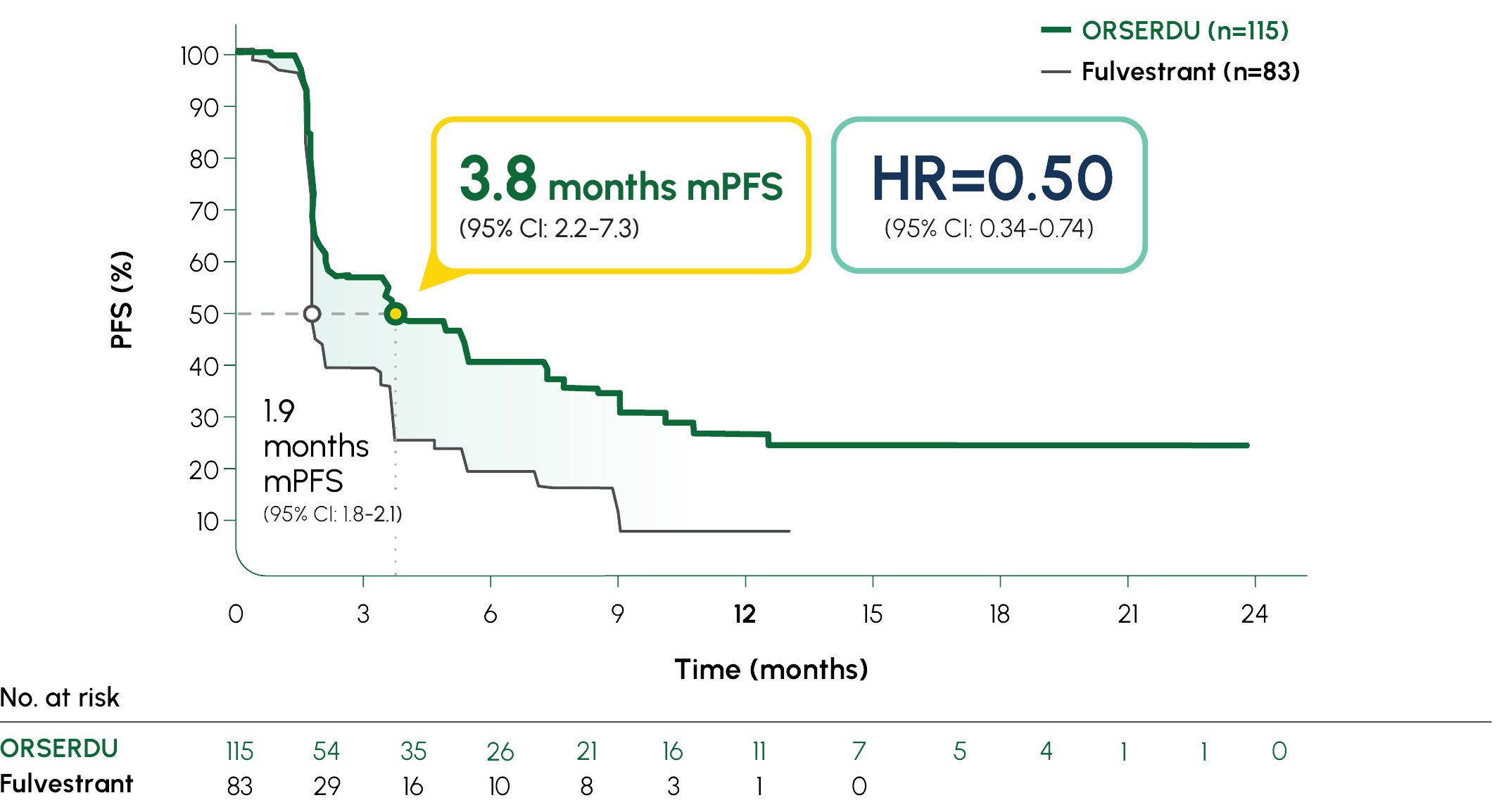
PRIMARY ENDPOINT IN EMERALD: mPFS IN PATIENTS WITH ESR1-MUTATED mBC1
-
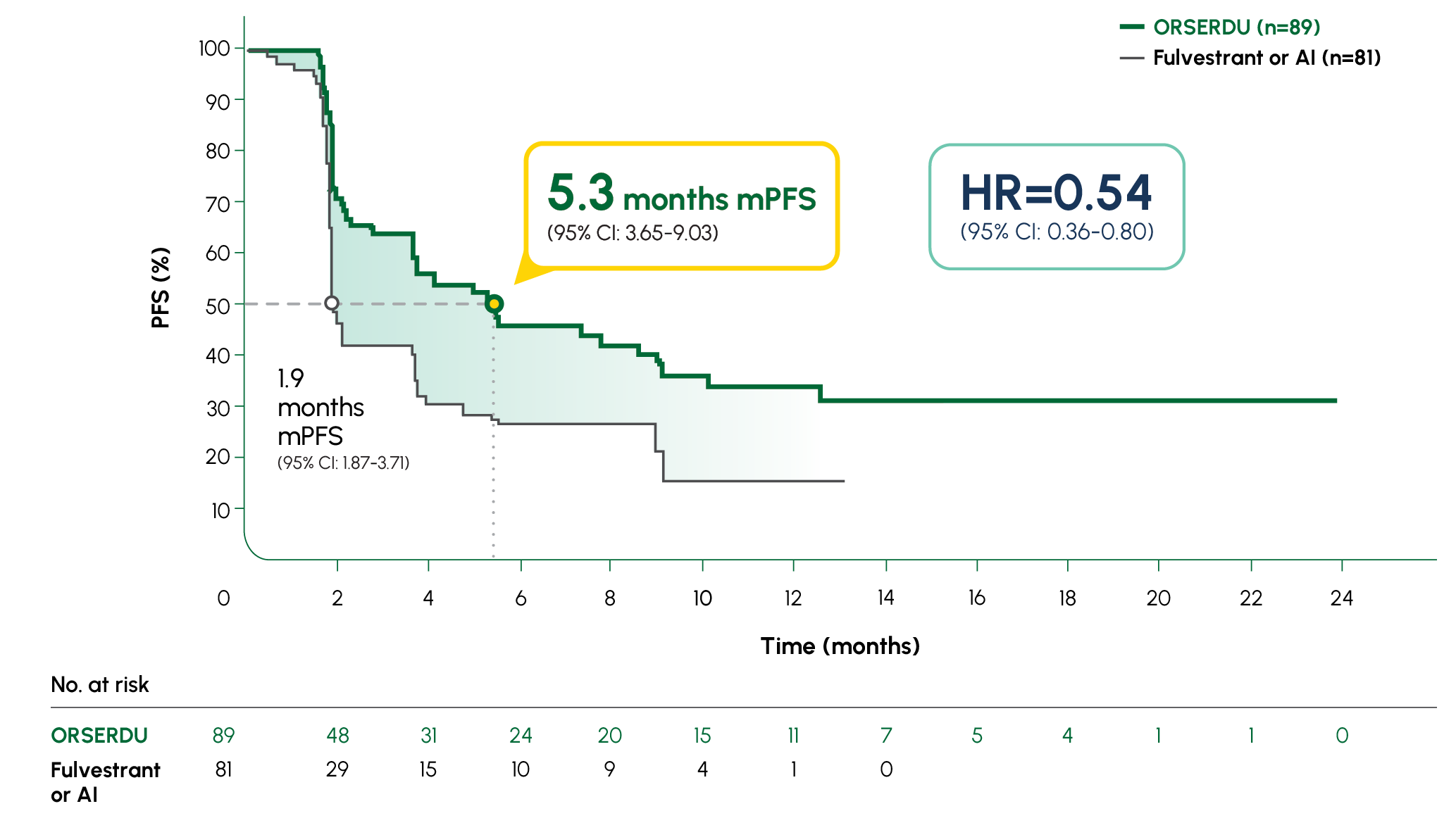
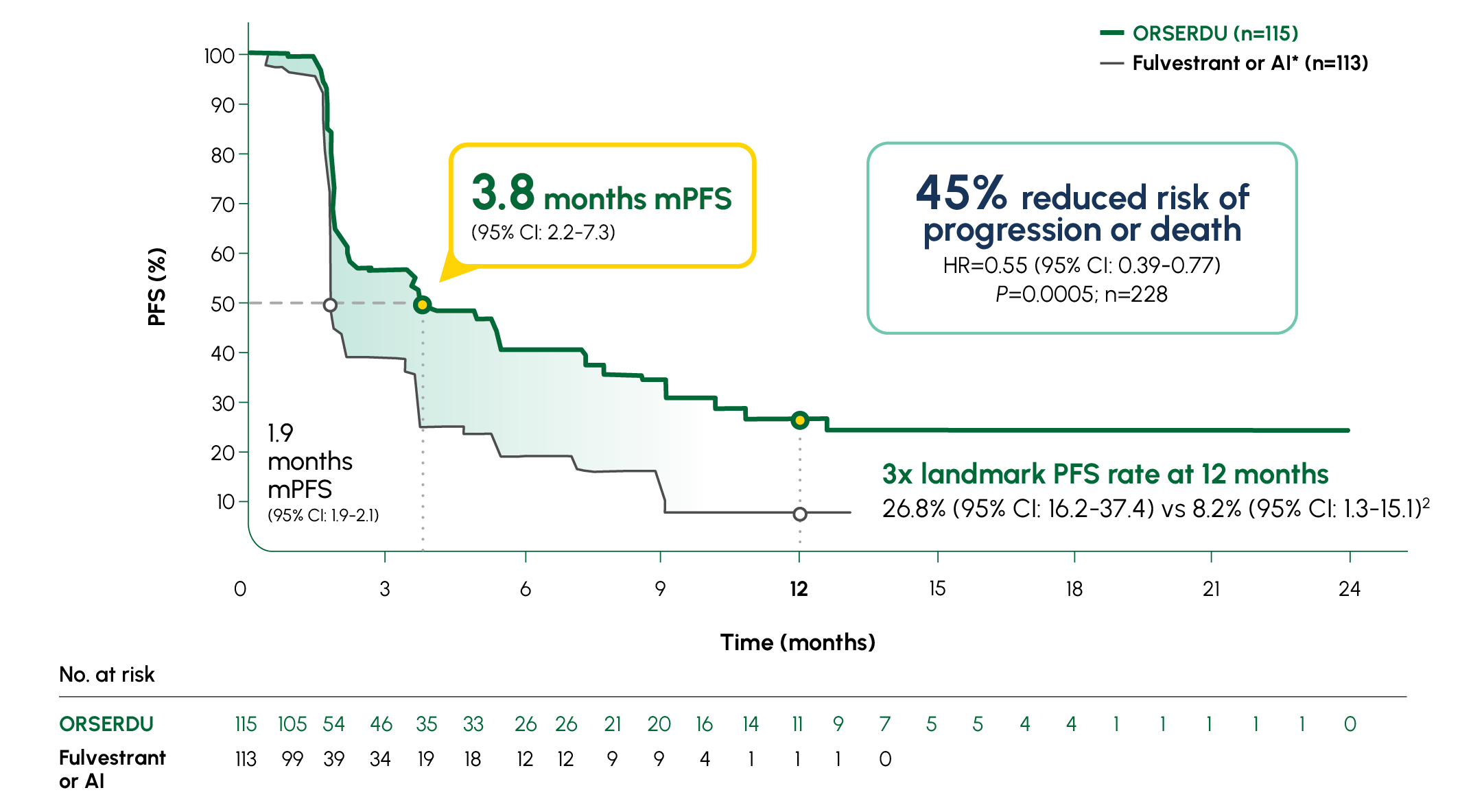
Number of PFS events: 62 (54%) with ORSERDU vs 78 (69%) with fulvestrant or AI1
-
A statistically significant PFS was observed in the ITT population and in the subgroup of patients with ESR1-mutated mBC. The exploratory PFS analysis in the 250 patients (52%) without ESR1 mutations showed a HR of 0.86 (95% CI: 0.63-1.19); thus, improvement in the ITT population was primarily attributed to the results seen in the patients with ESR1-mutated mBC1
-
Prespecified, descriptive analyses: PFS rates at 3, 6, 12, and 18 months are based on Kaplan-Meier estimates and are not powered to detect statistical difference at these time points. 3 months: ORSERDU 55.9% (95% CI: 45.8-66.1) vs fulvestrant or AI 39.6% (95% CI: 29.4-49.7); 6 months: ORSERDU 40.8% (95% CI: 30.1-51.4) vs fulvestrant or AI 19.1% (95% CI: 10.5-27.8); 12 months: ORSERDU 26.8% (95% CI: 16.2-37.4) vs fulvestrant or AI 8.2% (95% CI: 1.3-15.1); 18 months: ORSERDU 24.3% (95% CI: 13.7-35.0) vs fulvestrant or AI (NE)3
*AI therapy included anastrozole, letrozole, or exemestane.1

More than 12,000 patients with ESR1-mutated mBC have been treated with ORSERDU3

More than 5600 HCPs have prescribed ORSERDU to date3
AI, aromatase inhibitor; CI, confidence interval; ER+, estrogen receptor-positive; ESR1, estrogen receptor 1; ET, endocrine therapy; HER2-, human epidermal growth factor receptor 2-negative; HCP, healthcare professional; HR, hazard ratio; ITT, intent to treat; mBC, metastatic breast cancer; mPFS, median progression-free survival; NE, not estimable; PFS, progression-free survival.